Industry Lobbyists Work to Influence U.S. Position in Critical Global Health Negotiations
In October 2020, India and South Africa, recognizing the unprecedented urgency of the COVID-19 pandemic, proposed a temporary waiver from certain provisions of the World Trade Organization (WTO) Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) in order to ensure that intellectual property (IP) would not be a barrier to timely and affordable access to medical tools for COVID-19. The negotiations that followed this proposal amounted to over three years of discussions at the WTO that stand in stark contrast to the urgent action required to address the COVID-19 pandemic.
After an initial period in which the U.S. and other wealthy countries blocked productive negotiations on the waiver, in May 2021, U.S. Trade Representative Katherine Tai announced the Biden administration’s support for waiving IP provisions for COVID-19 vaccines—a welcome shift from the opposition shown under the Trump administration.
In June 2022, a limited waiver was adopted for COVID-19 vaccines. This decision relaxed a narrow band of requirements for “compulsory licensing” of vaccine patents, by which countries may authorize competition to support affordable and diverse vaccine supply. WTO members also committed to continue negotiations on whether to extend this decision on COVID-19 vaccines to therapeutics and diagnostics. Finally, in February 2024, the WTO officially declaredthat consensus could not be reached on the waiver extension.
Concurrently with the WTO waiver discussions, Member States of the World Health Organization (WHO) began negotiating a Pandemic Agreement, whose principal aim is to address the inequities observed during the global response to COVID-19. The Agreement could help foster international cooperation and coordination to address pandemics, including to avoid gridlocked talks at the WTO during pandemic emergencies.
The COVID-19 IP waiver proposal elicited an extensive lobbying effort from pharmaceutical companies and trade associations, including public ad campaigns claiming that the waiver would “eliminate” IP protections. The U.S., along with other high-income countries, have taken similar positions in the WHO Pandemic Agreement negotiations.
Public Citizen examined U.S. lobbying activity on the TRIPS waiver between 2021 and the first half of 2024. This data revealed an imbalance between those lobbying against the waiver compared to those lobbying in support. Additionally, lobbying disclosures show an opposition effort that extends well into 2024. We further examined U.S. lobbying activity on the ongoing negotiations at the WHO for a Pandemic Agreement.
Key Findings:
- More than 500 lobbyists were hired to lobby on the waiver between 2021 and the present. Of these, nearly 90% were hired by entities opposed to the waiver. Those hiring the most lobbyists were pharmaceutical and biotechnology companies or industry groups with pharmaceutical or biotechnology company-affiliated members.
- In 2022, the year in which the most lobbyists were hired, entities opposed to the waiver outnumbered those hired by supporters 32 to 1.
- Two dozen entities disclosed lobbying on the waiver through the first half of 2024 when COVID waiver talks concluded. The majority of these entities were pharmaceutical or biotechnology companies and the trade associations that represent them.
- Fewer entities have lobbied on the Pandemic Agreement. Entities included the Chamber of Commerce and the Biotechnology Innovation Organization, who hired dozens of lobbyists to influence the Pandemic Agreement negotiations.
Powerful Opposition to the TRIPS Waiver
Corporations, trade groups, and non-profits hired more than 500 lobbyists to lobby on the TRIPS waiver from 2021 through the second quarter of 2024.[1] Nearly 90 percent of these lobbyists were hired by entities opposed to the waiver.
In 2022, the year in which the most lobbyists were hired, entities opposed to the TRIPS waiver outnumbered those hired by the supporters 32 to 1. [Figure 1]
Figure 1. TRIPS Waiver Lobbyists
(by Year and Position)
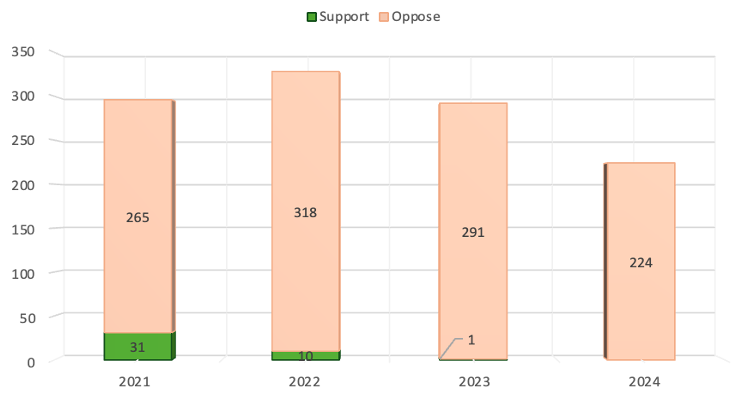
* Figure 1 includes only lobbyists for which Public Citizen was able to determine either clear support or opposition to the TRIPS waiver. We were able to determine a clear position for 95 percent of all lobbyists.
The lobbyist totals in Figure 1 include each unique client-lobbyist relationship – not each unique person. Lobbying clients will often hire the same people resulting in the same person being counted multiple times (for example, when Pfizer and PhRMA both hire the same person to lobby, that person would be counted twice in our totals).
From 2021 through the second quarter of 2024, 12 different entities hired 10 or more lobbyists to lobby on the TRIPS waiver. All 12 entities were opposed to the waiver. The Chamber of Commerce hired the most lobbyists, 72, followed by PhRMA (64), Pfizer (54), BIO (36), and Novartis (30). Of the top 20 entities hiring the most lobbyists to lobby on the TRIPS waiver, 18 were pharmaceutical or biotechnology companies or trade groups that represent them. [Table 1]
Table 1. Top 20 Lobbying Entities (by Lobbyist Count)
| 1 | *Chamber of Commerce[2] | 11 | *The Business Roundtable, Inc.[3] |
| 2 | *Pharmaceutical Research and Manufacturers of America (PhRMA)[4] | 12 | *Merck[5] |
| 3 | *Pfizer[6] | 13 | *Advanced Medical Technology Association (AdvaMed)[7] |
| 4 | *Biotechnology Innovation Organization (BIO)[8] | 14 | *AstraZeneca[9] |
| 5 | *Novartis[10] | 15 | *Eli Lilly[11] |
| 6 | *Amgen[12] | 16 | *Sanofi[13] |
| 7 | *Bristol-Myers Squibb[14] | 17 | *Bayer[15] |
| 8 | *GSK[16] | 18 | Motion Picture Association[17] |
| 9 | *Gilead Sciences[18] | 19 | *Johnson & Johnson[19] |
| 10 | *Genentech[20] | 20 | National Taxpayers Union[21] |
* Indicates pharmaceutical/biotechnology companies and trade groups with pharma/biotech industry members.
Sustained Opposition to the TRIPS Waiver
Pharmaceutical and biotech companies and the trade groups that represent them continued to lobby on the TRIPS waiver well into 2024. This despite the adoption of a much-diminished waiver for COVID-19 vaccines in 2022, followed by protracted discussions on whether to extend the waiver to cover diagnostics and therapeutics, and ultimately, an understanding in December 2023 that an extension was unlikely. It was subsequently no surprise when, in February 2024, the WTO officially declared that consensus on the waiver extension could not be reached.
Lobbying disclosures from the second quarter of 2024 show that 24 entities continue to disclose TRIPS waiver lobbying. Of these, the majority are pharmaceutical or biotech companies and the trade associations that represent them. [Table 2]
Table 2. Entities Disclosing Waiver Lobbying in 2024Q2 (Alphabetical)
| *Advanced Medical Technology Association (AdvaMed)[22] | *Gilead Sciences[23] |
| American Intellectual Property Law Association (AIPLA)[24] | *GSK[25] |
| *Amgen[26] | *Johnson & Johnson[27] |
| AUTM[28] | *Life Sciences Pennsylvania[29] |
| *Biocom[30] | *Eli Lilly[31] |
| *BIOGEN[32] | *Merck[33] |
| *Biotechnology Innovation Organization (BIO)[34] | *Novartis[35] |
| *California Life Sciences[36] | *Pfizer[37] |
| *Chamber of Commerce[38] | *Pharmaceutical Research and Manufacturers of America (PhRMA)[39] |
| Conservatives for Property Rights[40] | *Regeneron Pharmaceuticals[41] |
| Council For Innovation Promotion (C4IP)[42] | *Sage Therapeutics[43] |
| *Genentech[44] | *Sanofi[45] |
* Indicates pharmaceutical/biotechnology companies and trade groups with pharma/biotech industry members.
WHO Pandemic Agreement Lobbying
Pharma-aligned trade groups have also lobbied on the WHO Pandemic Agreement, an international instrument that aims to prevent the extreme inequity observed during the COVID-19 pandemic.
The most powerful trade group in the country, the U.S. Chamber of Commerce, which counts some of the largest pharmaceutical companies in the world as its members, including Pfizer,[46] Eli Lilly,[47] and Johnson and Johnson,[48] began lobbying on the Pandemic Agreement in 2021.[49] According to OpenSecrets, the Chamber spent close to $70 million lobbying on all issues in 2023, making it the largest lobbying spender in the country.[50] In 2024, the trade group sent 49 lobbyists to Capitol Hill to lobby on the Pandemic Agreement.[51] BIO, another powerful trade group with many pharmaceutical company members,[52] deployed 16 lobbyists to influence the Pandemic Agreement negotiations, according to its 2024 second quarter lobbying disclosure.[53]
Although lobbying disclosures do not specify positions on the Pandemic Agreement, both the Chamber and BIO have remarked on the Agreement in public comments. Such comments claim a supposed threat to the global IP system from the WTO waiver talks and IP provisions in the Pandemic Agreement, praise pro-industry U.S. negotiating positions, and voice opposition to common sense public interest provisions that call for government-funded research and development agreements to be more transparent and facilitate equitable access to pandemic-related medical tools.
In the midst of COVID inequity, the WTO waiver negotiations denoted a failure of emergency response. One marked by entrenched positions, stalled negotiations, and inaction by the WTO. It is critical that the Pandemic Agreement does not fail similarly. To achieve this, wealthy countries must ensure that productive negotiations are not inhibited by industry influence.
[1] The lobbyists total counts the same lobbyist that worked for multiple different entities, multiple times. Essentially, the total amounts to each unique lobbyist-client relationship.
[2] https://lda.senate.gov/filings/public/filing/4ebff9cb-1435-4329-ac7e-bd29f6ab05a3/print/ (Disclosing lobbying); https://www.pfizer.com/about/programs-policies/political-partnerships and https://www.lilly.com/policies-reports/public-policy-political-participation (Showing pharmaceutical company members Pfizer and Eli Lilly).
[3] https://lda.senate.gov/filings/public/filing/34e8508a-149e-45dd-9760-f6560907cb1f/print/ (Disclosing lobbying); https://www.businessroundtable.org/about-us/members (Showing pharma-affiliated members).
[4] https://lda.senate.gov/filings/public/filing/b587fd57-3119-43e4-9c50-1de2ce37e072/print/ (Disclosing lobbying); https://phrma.org/en/About (Showing pharmaceutical company members).
[5] https://lda.senate.gov/filings/public/filing/1d7187b8-8b2a-4aa5-940c-17f845a80185/print/ (Disclosing lobbying).
[6] https://lda.senate.gov/filings/public/filing/3eed53ac-120d-42a3-854c-6c0c549213b1/print/ (Disclosing lobbying).
[7] https://lda.senate.gov/filings/public/filing/595ee5e0-aa2d-463d-ad54-c10b568e4855/print/ (Disclosing lobbying); https://www.advamed.org/membership-join/membership-directory/ (Showing pharmaceutical/biotech members).
[8] https://lda.senate.gov/filings/public/filing/648ce811-86d9-4a96-9234-f4295d0b7cdc/print/ (Disclosing lobbying); https://www.bio.org/member/bio-member-directory (Showing pharmaceutical/biotech members).
[9] https://lda.senate.gov/filings/public/filing/5f91ebda-613c-4f00-b8db-8c880262e65d/print/ (Disclosing lobbying).
[10] https://lda.senate.gov/filings/public/filing/787ce39f-94f6-476a-8f8c-1f984cced1fe/print/ (Disclosing lobbying).
[11] https://lda.senate.gov/filings/public/filing/79eacf65-134e-4cc2-9ed3-4267071be73d/print/ (Disclosing lobbying).
[12] https://lda.senate.gov/filings/public/filing/9645b3c1-9372-44ec-b973-84a13e5cebea/print/ (Disclosing lobbying).
[13] https://lda.senate.gov/filings/public/filing/c39981bf-40b1-4b52-b921-70bb213e2052/print/ (Disclosing lobbying).
[14] https://lda.senate.gov/filings/public/filing/d43d83dc-54b1-4cce-91c1-1041d12196c0/print/ (Disclosing lobbying).
[15] https://lda.senate.gov/filings/public/filing/07f51df1-1fba-43c9-9b3e-a5d9f2d9c8ec/print/ (Disclosing lobbying).
[16] https://lda.senate.gov/filings/public/filing/ae02e501-b337-45d0-b87e-1c6bb9f99be0/print/ (Disclosing lobbying).
[17] https://lda.senate.gov/filings/public/filing/ae41dbc9-8f30-47cd-86b9-e6e1c5282881/print/ (Disclosing lobbying).
[18] https://lda.senate.gov/filings/public/filing/94c3aa7e-ae6e-4ce1-860c-c6290c6823e4/print/ (Disclosing lobbying).
[19] https://lda.senate.gov/filings/public/filing/21019502-1300-4eee-bc3a-0cff8c4ab682/print/ (Disclosing lobbying).
[20] https://lda.senate.gov/filings/public/filing/c80d7b4d-ee5a-46bc-955d-bc9879c425c1/print/ (Disclosing lobbying).
[21] https://lda.senate.gov/filings/public/filing/ff21404e-c298-4364-8d88-4b0679c245cf/print/ (Disclosing lobbying).
[22] https://lda.senate.gov/filings/public/filing/76e3ade4-5e70-4b69-8b07-bf9e59f5fbda/print/ (Disclosing lobbying).
[23] https://lda.senate.gov/filings/public/filing/24397104-c614-4b4a-8e1c-5e5b4fd3f2e0/print/ (Disclosing lobbying).
[24] https://lda.senate.gov/filings/public/filing/6af431af-16f3-46f4-8194-e7cef5709124/print/ (Disclosing lobbying).
[25] https://lda.senate.gov/filings/public/filing/ce8426aa-42f5-4f07-80f8-678923e1daa3/print/ (Disclosing lobbying).
[26] https://lda.senate.gov/filings/public/filing/6cb41732-f98c-4ac9-948f-699318899057/print/ (Disclosing lobbying).
[27] https://lda.senate.gov/filings/public/filing/892c5512-a57d-482f-aedb-86ee9c7d45f6/print/ (Disclosing lobbying).
[28] https://lda.senate.gov/filings/public/filing/b0fbad15-0c16-4be3-a9df-be07702f9fb8/print/ (Disclosing lobbying).
[29] https://lda.senate.gov/filings/public/filing/cef58e3f-d766-4aac-b688-afd31a41ac9b/print/ (Disclosing lobbying).; https://members.lifesciencespa.org/directory/Search/pharmaceutical-315656 (Showing pharma/biotech members).
[30] https://lda.senate.gov/filings/public/filing/e69d83a0-7179-4fd6-a22f-034c30d6ec4f/print/ (Disclosing lobbying); https://www.biocom.org/members/ (Showing pharma/biotech members).
[31] https://lda.senate.gov/filings/public/filing/e07695aa-bedc-4308-b75b-1927e65f5f8e/print/ (Disclosing lobbying).
[32] https://lda.senate.gov/filings/public/filing/401d508d-051d-46fe-a9ef-f3f5d2f18523/print/ (Disclosing lobbying).
[33] https://lda.senate.gov/filings/public/filing/6df05a18-1eb6-4de6-ac4e-322509189580/print/ (Disclosing lobbying).
[34] https://lda.senate.gov/filings/public/filing/991dd04b-1c9c-48bf-a6de-1cff187913c7/print/ (Disclosing lobbying).
[35] https://lda.senate.gov/filings/public/filing/3d07898e-326d-41b2-9d7b-96d12e7f9c44/print/ (Disclosing lobbying).
[36] https://lda.senate.gov/filings/public/filing/a1b7ba32-7c22-427d-9620-71add7d9992a/print/ (Disclosing lobbying); https://www.abbott.com/investors/governance/corporate-political-participation.html; https://www.amgen.com/about/how-we-operate/policies-practices-and-disclosures/transparency-disclosures/political-contributions
[37] https://lda.senate.gov/filings/public/filing/2b812d9b-8024-4ec5-9361-6f41a2f2b17a/print/ (Disclosing lobbying).
[38] https://lda.senate.gov/filings/public/filing/93fcbf8c-5a8f-4a15-a03b-d28a440a68ae/print/ (Disclosing lobbying).
[39] https://lda.senate.gov/filings/public/filing/22e6f546-e5dd-4daa-8f3b-a9361e40df52/print/ (Disclosing lobbying).
[40] https://lda.senate.gov/filings/public/filing/47c1c811-c6c7-4403-943b-4d30e105bac3/print/ (Disclosing lobbying).
[41] https://lda.senate.gov/filings/public/filing/1cc17aaa-46af-460c-bdb8-afa98cfc210c/print/ (Disclosing lobbying).
[42] https://lda.senate.gov/filings/public/filing/cc549722-01a6-4373-a5ef-9af01a49e239/print/ (Disclosing lobbying).
[43] https://lda.senate.gov/filings/public/filing/18373fc8-df36-458a-b8ba-535bfbb1ca01/print/ (Disclosing lobbying).
[44] https://lda.senate.gov/filings/public/filing/3077b351-44c9-469c-a551-12e58485cf6b/print/ (Disclosing lobbying).
[45] https://lda.senate.gov/filings/public/filing/e5c867fd-c497-48e6-8959-fae322c04778/print/ (Disclosing lobbying).
[46] https://www.pfizer.com/about/programs-policies/political-partnerships
[47] https://www.lilly.com/policies-reports/public-policy-political-participation
[48] https://www.jnj.com/about-jnj/policies-and-positions/our-position-on-stakeholder-engagement
[49] https://lda.senate.gov/filings/public/filing/87063175-dcae-41b3-863e-1609d8bea01d/print/
[50] https://www.opensecrets.org/federal-lobbying/top-spenders?cycle=2023
[51] https://lda.senate.gov/filings/public/filing/4ebff9cb-1435-4329-ac7e-bd29f6ab05a3/print/
[52] https://www.bio.org/member/bio-member-directory
[53] https://lda.senate.gov/filings/public/filing/991dd04b-1c9c-48bf-a6de-1cff187913c7/print/