Covax’s Choices
On January 17, 2020, as a mysterious new coronavirus spread through China, the Coalition for Epidemic Preparedness Innovations (CEPI) convened its advisers. CEPI, a global non-profit, was less than a week away from announcing investments to jumpstart the development of three coronavirus vaccine candidates.[1] At the meeting, the members of the Equitable Access Committee made their long-standing case. Affordability and transparency were critically important, they said.[2] CEPI needed to communicate more clearly its “focus on stewardship and public goods goals.”[3] The organization should also be clearer about how its equitable access policy is implemented “in a way that has teeth.”[4]
Ten months later, as the virus continues to devastate countries, those goals have taken on a profound importance. To promote vaccine access, CEPI has now joined forces with Gavi, the Vaccines Alliance, and the World Health Organization as part of a partnership called COVAX.[5] COVAX aims “to accelerate the development and manufacture of COVID-19 vaccines, and to guarantee fair and equitable access for every country in the world.”[6] Building off CEPI’s early-stage investments in nine vaccine candidates, COVAX seeks to place advance orders with vaccine manufacturers to secure doses.[7] The success of COVAX depends in large part on the details of its deals: How much vaccine will be available, at what price, by when?
In this report, we analyze a previously unreported mechanism that could help COVAX achieve its ambition to rapidly supply the world with an affordable vaccine—so long as it is willing to show its teeth. COVAX includes the candidates CEPI has funded in its vaccine portfolio. CEPI, as a critical funder of medical technology, has rights to see it used responsibly in the world.[8] We examine equitable access conditions that CEPI has included its contracts. The precise contours of the conditions remain unclear due to a lack of transparency. But we find in the cases of:
- CureVac: CEPI appears to retain a “public health license” and an oversight authority on the how technology it funds is used. The public health license could allow CEPI, in some circumstances, to require the corporation to share vaccine technology, and allow additional qualified suppliers. The oversight authority requires CureVac to obtain CEPI’s consent to use the technology it funds in a way that goes against CEPI policies, and could potentially be used to restrict vaccine hoarding;
- Novavax and University of Queensland: CEPI appears to retain a public health license and “step-in rights”, respectively;
- Moderna: The corporation appears to be violating CEPI’s principle to provide access based on public health need, instead of ability to pay; and
- Clover Biopharmaceuticals, Oxford (AstraZeneca), Themis (Merck), University of Hong Kong: There is some evidence to suggest that they may have committed “to transfer their vaccine technology to a global network of large-scale manufacturers.” The scope of this obligation remains unclear.
We proceed in three parts. First, we provide an overview of COVAX and describe potential challenges. Second, we review CEPI contracts and, where data is available, analyze the public interest safeguards. Finally, we conclude by charting a course forward.
No one corporation can supply the world with a vaccine. CEPI—and COVAX—should make all their contracts publicly available, and enforce corporate compliance with their equitable access conditions. They should also leverage the full extent of their existing authority to increase technology transfer and scale-up affordable supply. Moving forward, they should coordinate with the WHO’s sister initiative, the COVID-19 technology access pool, to openly share technology, so that all qualified manufacturers are able to scale-up access.[9]
Neither vaccine doses, nor the knowledge needed to produce vaccines, should be hoarded. That is the only way to make vaccines a global public good.
Covax: A Vaccine Partnership
A collaboration of CEPI, Gavi and WHO, COVAX is the vaccines pillar of the Access to COVID-19 Tools (ACT) Accelerator. COVAX aims to provide two billion doses of vaccines by the end of 2021. Building on CEPI’s early-stage investments, COVAX seeks to place advanced orders with vaccine manufacturers through a financial instrument called the “COVAX Facility.” Gavi and CEPI are collaborating on the design and operation of the Facility.
The Facility comprises of two distinct streams for Self-Financing Countries, and Advance Market Commitment (AMC) countries. Self-financing countries include high income and upper-middle-income countries.[10] AMC countries include 92 lower and middle income countries, whose participation will be supported in part by donor funding.[11] The two billion target doses will be divided between the two streams, with some set aside for emergency deployment.
Figure 1: COVAX slide presented to Member States at the World Health Organization (August 13, 2020).[12]
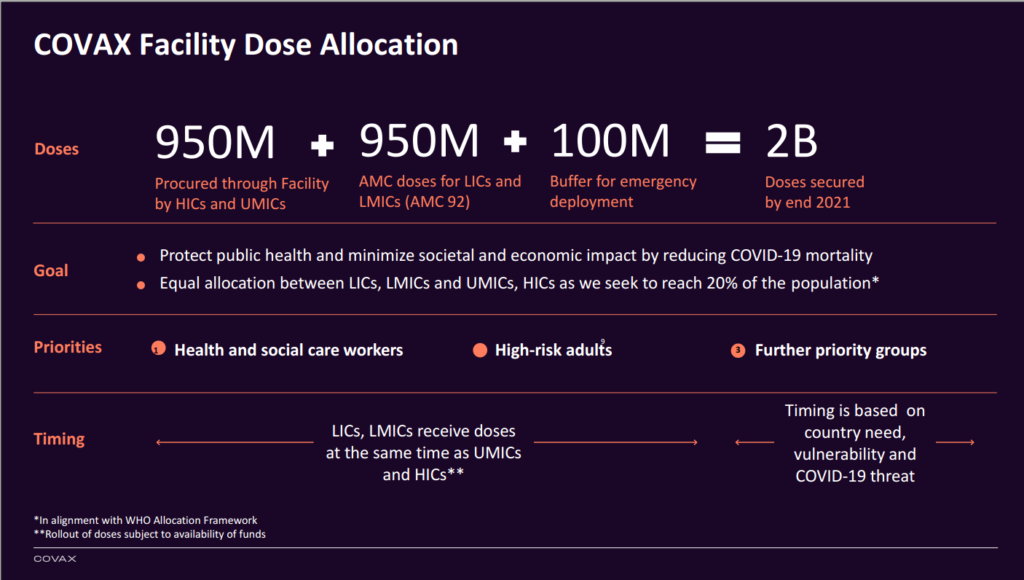
In line with the World Health Organization’s equitable allocation framework, doses will initially be allocated to cover 20 percent of the population in participating countries, as long as funding requirements are met.[13] COVAX participants will also have the option to trade their vaccine allocation with other countries.
The current design of the Facility raises at least two challenges.
First, vaccine supply may be artificially restricted.[14] COVAX currently projects to supply two billion doses by the end of 2021, and then scale-up over time. The goal is ambitious, but also less than necessary to meet global demand. Researchers estimate several times as many doses may be needed—with demand only increasing if the vaccines provide limited immunity and require regular booster shots.[15] The Facility, however, is currently only “encouraging” manufacturers to share technology and scale-up supply among a limited pool of suppliers.[16] The Facility justifies the voluntary approach based on the complex nature of technology transfer. But limited sharing, dictated by market access considerations, may fail to make full use of all available global production capacity. This may lead to artificial scarcity. Such a scenario could be especially concerning if only a small number of candidates succeed and/or demonstrate clear superiority to others in clinical trials, and the successful manufacturers refuse to widely share technology to scale-up supply. (This could also lead to a “rush for the best”, which may leave low-and-middle income countries with access to inferior products, if any—a possibility only exacerbated by a COVAX trading exchange.)
Second, vaccines may be priced out of reach. COVAX projects to supply doses at an average cost of $10.55 per dose—likely $21.1 per course—for self-financing participants.[17] Governments are signing up for the Facility and making upfront payments based on that estimate. But COVAX has provided no public assurances that the vaccines will actually be sold by manufacturers to governments close to that price.[18]
Self-Financing Countries’ Binding Commitment = Average estimated cost per dose ($10.55) x Expected doses per regimen (2) x Agreed proportion of participants’ population
The Facility only says it will “endeavor to negotiate the best possible pricing from manufacturers that is lower than or at least no higher than pricing manufacturers have agreed in bilateral deals.”[19] While countries will only remain financially liable for their initial investment, it remains unclear whether countries will get the doses they need with the amount they invest right now.
This is particularly concerning because a small number of candidates may be driving down the average estimated cost of a vaccine right now. For example, COVAX procured the Oxford-AstraZeneca vaccine for around $2.5 per dose, which is the among the lowest publicly reported prices for a vaccine.[20] If that candidate fails, the average price of the COVAX portfolio would likely increase.
There is a better way. COVAX is not merely a final purchaser. Through CEPI, it has also funded research, development, and manufacturing. COVAX could demand more of manufacturers and fully use the leverage provided by CEPI’s critical early-stage funding to share technology and scale-up affordable supply.
CEPI’s Commitments
The Coalition for Epidemic Preparedness Innovations was created in response to the West Africa Ebola Outbreak by public and philanthropic institutions. It has since played a vital role in advancing new vaccines and new vaccine platform technologies. Since January, CEPI has invested $1.1 billion in nine COVID-19 vaccine candidates, often at the riskiest stages of their development.[21] Eight have entered clinical trials.[22] CEPI is working to raise billions of dollars to make additional investments in the candidates.[23]
Table 1: CEPI COVID-19 Vaccine Contracts
| Vaccine Candidate | Platform | Phase | CEPI Funding | Announcement |
| Inovio | DNA | I/II | $22.5 million | 1/23/20[24] , 4/16/20 |
| Moderna | mRNA | III | $1 million[25] | 1/23/20 |
| University of Queensland (CSL) | Protein | I | $4.5 million | 1/23/20[26], 6/5/20 |
| CureVac | mRNA | II | $15.3 million | 1/31/20 |
| Novavax | Protein | III | $388 million | 3/10/20 , 5/11/20 |
| University of Hong Kong | Viral Vector | Preclinical | $0.62 million | 3/18/20 |
| Themis/Institut Pasteur/University of Pittsburgh (Merck) | Viral Vector | I | $4.9 million | 3/19/20 |
| Oxford (AstraZeneca) | Viral Vector | III | $384.1 million | 3/10/20, 6/4/20 |
| Clover Biopharmaceuticals | Protein | I | $328 million | 4/27/20, 11/3/20 |
CEPI beneficiaries are required to abide by its equitable access policy.[27] The policy lays out general access principles, affirming CEPI’s commitment to equitable access and providing that “equitable access to epidemic vaccines in the context of an outbreak means that appropriate vaccines are first available to populations when and where they are needed to end an outbreak or curtail an epidemic, regardless of ability to pay.” [28] (emphasis in original)
CEPI includes access considerations in all partnership agreements.[29] The precise provisions included in each contract vary based on the negotiation.[30] A CEPI Template Agreement provides one example of how the access policy generally binds awardees:
The Awardee will: to the extent that Awardee commercializes Product which utilizes or otherwise benefits from, whether directly or indirectly, any Project Result, the distribution of that product in LMICs will be consistent with CEPI’s Equitable Access Policy and Cost Guidance Policy, as well as with The Bill & Melinda Gates Foundation’s approach to determining appropriate product costs.[31]
These provisions could be used to help expand affordable supply.
To identify the specific equitable access provisions applicable to the COVID-19 candidates, we reviewed publicly available documents related to vaccine development from CEPI, including organizational policies, press releases, application proposals, and meeting minutes. We also reviewed financial statements from the corporations who received CEPI funding for COVID-19. Our analysis is limited by a lack of transparency. We were able to obtain only two CEPI contracts (i.e., with Novavax and CureVac). For the other candidates, we relied on a combination of statements in other documents.
CureVac
Overview
In January, CEPI provided CureVac with up to $15.3 million in funding for preclinical development and a Phase I trial.[32] Previously, CEPI had invested $34 million in CureVac’s RNA Printer platform technology in 2019.[33]
Equitable Access
CEPI’s partnership with CureVac on the coronavirus candidate vaccine built off their original partnering agreement.[34] The same equitable access conditions appear to apply.[35] Details about the new work package are otherwise heavily redacted. We document some safeguards present in the original agreement below, and then analyze how they could be used to expand affordable supply.[36]
Table 2 – Select Public Interest Safeguards in CureVac – CEPI Contract [37]
| Public Interest Safeguard | Excerpt |
| Transparency about production, supply, pricing and sales, and right to audit
|
Article 7.11.9. Equitable Access.
“Subject to Work Packages, Partner shall provide the following to CEPI to the extent not already included in the reports and information provided by the Partner to CEPI: (i) progress report on the scale-up of the Platform for Manufacturing and a good faith estimate of the cost of the scale-up where such scale-up is necessary; Article 3.16. Audit by CEPI. “The Partner shall provide access to the Partner Financial Records to auditors and other personnel from or appointed by CEPI. . .” |
| Expanding supply through Trusted Manufacturers | Article 7.6. Trusted Manufacturers.
“Subject to the undertakings to be defined in the Additional Work Packages and – upon Partner’s request, subject to a separate confidentiality agreement to be concluded between the Partner and the Trusted Manufacturer – the Partner will support CEPI in appointing one or more Trusted Manufacturers that are technically and operationally capable of and willing to rapidly Manufacture Product for use in the Field in the Affected Territory on an ongoing basis both during and after completion of the Project, in accordance with CEPI’s requirements, as set forth herein. 7.6.1.Subject to the undertakings in the Additional Work Packages the Partner shall: (i) grant appointed Trusted Manufacturers all necessary rights to use (on a non-exclusive, royalty-free and license-fee free basis) the Background Technology and Project Technology. . .; (ii) provide the Technology Transfer Materials to the Trusted Manufacturers and ensure that such Technology Transfer Materials are kept up to date . . .”[38] |
| Public Health License | Article 11.1. Public Health License.
“The Partner hereby grants to CEPI with effect from the Effective Date, a non-exclusive, worldwide, royalty-free and license-fee free license (except in respect of the sharing of Commercial Benefits pursuant to Clause 13) under the Background Technology and Project Technology to: 11.1.1. develop the Platform for use in the Field via Trusted Manufacturers; (together, the “Public Health License”); provided however that CEPI may not exercise the rights granted under the Public Health License unless and until the occurrence of one or more Conditions Precedent.” Article 12.1. Conditions Precedent and Exercise of the Public Health License. “CEPI may exercise the Public Health License by notice in writing to the Partner on the occurrence of one or more of the events set out below (each a “Condition Precedent” and together the “Conditions Precedent”): 12.1.4. if by the date [*****] following successful completion of the Project the Cost of Goods for the Project Vaccines for use in the Field exceeds the level public service agencies agree is affordable based on objective economic criteria to be determined between the Parties for use in the Affected Territory; . . .” Article 12.3. Effects of exercise of the Public Health License. “On exercise of the Public Health License, CEPI, after consultation with Partner, shall have the discretion to make any reasonable decisions in relation to the Development of the Platform for use in the Field, the Development of Products including Project Vaccine, Manufacturing and marketing of the Product for use in the Field in the Affected Territory by the Trusted Manufacturer(s). The Partner shall use all reasonable endeavors to give assistance to CEPI and/or the Trusted Manufacturer(s) in relation to such Manufacturing for use in the Field in the Affected Territory including executing any necessary documents.” Article 12.5. Release of Technology Transfer Materials. On the exercise of the Public Health License, the Partner shall release immediately the Technology Transfer Material[40] |
| Oversight authority | Article 15.1. Partner’s Undertakings.
To the extent it is contractually able to do so, Partner shall obtain CEPI’s prior written consent before exploiting, or allowing a Third Party to exploit any of the Project Technology within the Field, provided the exploitation is in conflict with or goes against CEPI’s mission, the CEPI Policies or the provisions of this Clause.[41] |
Implications
CEPI has rights in the RNA Printer platform and the CureVac coronavirus candidate. First, CEPI appears to have a powerful oversight authority. CureVac is required to obtain CEPI’s prior written consent before using in the field technologies developed under the agreement if the use conflicts with or goes against CEPI’s policies.[42] In a financial filing, CureVac affirms this interpretation noting that it is “required to obtain CEPI’s consent prior to exploiting any intellectual property developed under the CEPI Agreement if such exploitation is in conflict with or goes against CEPI’s mission or policies.”[43]
Chief among these policies is the Equitable Access policy. It lays out CEPI’s general commitments, including its principle that
Equitable access to epidemic vaccines in the context of an outbreak means that appropriate vaccines are first available to populations when and where they are needed to end an outbreak or curtail an epidemic, regardless of ability to pay.[44] (emphasis in original)
CureVac has not publicly announced any deals. But it has said that it is in discussion with the European Commission “to provide all EU Member States with up to 225 million doses and an option for an additional purchase of 180 million doses.”
If and when this deal is finalized, it may not be consistent with CEPI’s policies. Equitable access is based on three conditions 1) timing (first available); 2) medical need (where they are needed to end an outbreak or curtail an epidemic); and 3) financial neutrality (regardless of ability to pay). Consider, for example, if CureVac predominantly supplied its vaccine to high-income countries, even as others faced more severe outbreaks. In that scenario, CureVac would appear to prioritize ability to pay over public health need. CureVac would also violate the distributional principles developed by WHO, CEPI and GAVI specifically for COVAX vaccine access, which require proportional allocation to meet the initial basic needs of all countries.[45]
If CureVac asked CEPI for its consent, CEPI could presumably deny the request.[46] CureVac could then proceed with the sale at risk of breaching the agreement, or attempt to come to a resolution. Under the contract, English courts have exclusive jurisdiction over this kind of claim.[47] (If CureVac failed to ask CEPI for its consent, CEPI could still likely bring a claim for a breach of the agreement, or terminate the agreement.[48])
Second, in addition to this general oversight authority, subject to the additional work package agreed to by CEPI and CureVac, CEPI has the ability to pursue appointment of other manufacturers (“Trusted Manufacturers”).[49] CureVac can veto a particular manufacturer, but final decisions on the number and identity of manufacturers can be appealed to an arbitrator.[50] Once appointed, CureVac is required to grant the Trusted Manufacturers all necessary rights to manufacture products for use, and provide technology transfer. Depending on the details of the additional work package, this means that CEPI could potentially open up supply for CureVac’s coronavirus vaccine candidate.
CEPI also has a worldwide, royalty-free license (“Public Health License”) to develop the coronavirus vaccine candidate, and manufacture and market the product via Trusted Manufacturers if certain conditions are met.[51] This authority is not explicitly subject to an additional work package.[52] For example, CEPI can exercise the license if the vaccine is not available in appropriate quantities and/or at an appropriate cost (though those terms remain undefined). Using these rights would further expand CEPI’s authority—even beyond merely suggesting Trusted Manufacturers—giving it the ability to make more decisions surrounding clinical development, supply and price.[53]
So far, CureVac has not appeared to publicly share technology with other suppliers. [54] CEPI should use its powerful authority to expand affordable supply for the CureVac candidate.
Novavax
Overview
CEPI provided Novavax funding for its coronavirus candidate, NVX-CoV2373, in March ($4.4 million) and May ($384 million).[55] Together, the funding aimed to help Novavax accelerate development and manufacture of its candidate, including by funding preclinical studies; phase 1 and 2 trials; technology transfer to manufacturing partners; and large-scale production of the Novavax’ adjuvant and candidate.
In May, CEPI said it anticipated “supporting any further clinical development needed to take NVX-CoV2373 through to licensure.” It is unclear how this interacts with the U.S. government’s subsequent $1.6 billion award to Novavax.[56]
Equitable Access
While some key terms remain redacted, CEPI has rights in the Novavax candidate. Many are included under the provision of an “Equitable Access Plan.” We document some safeguards below, and then analyze how they could be used to expand affordable supply.
Table 3 – Select Public Interest Safeguards in Novavax-CEPI Contract [57]
| Public Interest Safeguard | Excerpt |
| Public Health License | Article 13.4. Public Health License.
“Subject to the terms of this Agreement, Awardee hereby grants a worldwide and royalty free Public Health License to CEPI, on the condition that CEPI may only exercise the rights granted under the Public Health License in the event that: (b)the Project Vaccine has achieved licensure with at least one regulatory body (including but not limited to emergency licensure); and (c) one or more of the triggers set out in Clause 13.5 has occurred. CEPI shall be entitled to sublicense Project Results, Enabling IP and Background IP included in the Public Health License in accordance with this Clause 13 . . .”[58] Article 13.5. Public Health License Triggers. “Consistent with Clause 13.4, CEPI’s right to exercise the Public Health License shall be satisfied when: (a)Awardee declines to participate in an Additional Work Package or Project Expansion as requested by CEPI, either directly or indirectly through a Subawardee; (b)CEPI and Awardee agree, in good faith, that Awardee shall not be able to perform the activities under an agreed Work Package, either directly or indirectly through a Sub awardee; (c)Awardee is in material breach of this Agreement or the Equitable Access Plan and has not cured such breach within [***] days of notification of such breach by CEPI unless otherwise mutually agreed; or (d)the Agreement is terminated by CEPI pursuant to Clause 19.2(a)-(b) (default or insolvency) or 19.3(c) – (e) (unavailability to perform Project activities, failure to satisfy payment criteria or fraud).” Article 13.6. Agreement between CEPI and the Trusted Collaborator or Trusted Manufacturer. “. . . At CEPI’s request, Awardee shall use [***] to facilitate the conclusion of a direct contractual relationship between the Trusted Collaborator or Trusted Manufacturer, as the case may be, and CEPI. If those negotiations do not result in an agreement in [***], then CEPI may grant rights under its Public Health License to a third party unilaterally designated by CEPI as a Trusted Collaborator or Trusted Manufacturer, without approval from Awardee.” Article 13.7. Effects of Exercise of the Public Health License. “Upon exercise of the Public Health License and written notice to Awardee, Awardee [***] shall: . . . (c)[***] transfer to the Trusted Collaborator and/or Trusted Manufacturer, as the case may be, and at CEPI’s reasonable cost, all Project Results, Project Materials described in Clause 13.2(b), all guidance, information, materials and assistance reasonably required to accomplish the Project activities identified by CEPI; and (d)shall be deemed to have covenanted not to sue CEPI or designee for the exercise of the Public Health License.” |
| Reasonable Pricing
(Part of the Equitable Access Plan) |
Article 14.6. Pricing Objectives.
“Awardee agrees that its pricing shall be reasonable to achieve Equitable Access for populations in need of a Project Vaccine as well as an appropriate return on investment for vaccine manufacturers that make on-going supply commercially sustainable. . . For clarity, the purchase of Project Vaccine by the Global Allocation Body or by any other purchasing agent(s) designated by CEPI shall be considered to have satisfied the pricing requirements for Equitable Access.” |
| Requirement to negotiate an additional supply contract with COVAX and sell produced doses
(Part of the Equitable Access Plan) |
Article 14.3. Global Allocation.
“Awardee, will negotiate, in good faith a separate agreement or purchase order to supply Project Vaccine as may be required by the Global Allocation Body in such agreement or purchase order to the Global Allocation Body during the Pandemic Period and after the Pandemic Period for LMICs.” Article 14.4. Pandemic Period Production and Supply.[59] During the Pandemic Period, Awardee shall: (a)produce Project Vaccine as described in the Work Package(s), if not greater; . . . (d)supply up to [***] of the quantity of the Project Vaccine produced for purchase by the Global Allocation Body pursuant to Clause 14.3 during the Pandemic Period. For clarity, Awardee may not allocate or obligate Project Vaccine doses to other third parties during the Pandemic Period that conflicts with its obligations under this Clause 14 . . .“ |
| Transparency about production, supply, pricing and sales, and right to audit
(Part of the Equitable Access Plan) |
Article 14.8. Information about Production, Supply, Pricing and Sales.
“Upon written request by CEPI, Awardee shall provide reasonable information about its COGs, production, supply, pricing and sales of Project Vaccine sufficient to evaluate whether such activities meet the Equitable Access Policy.” Article 14.9 Audit of Cost of Goods. “No more than [***] and at CEPI’s reasonable cost, CEPI shall have the right to review or to designate an external auditor (which shall be an internationally recognized certified public accounting firm, not engaged on a contingent basis) to review Awardee’s financial records relevant to the information provided in Clause 14.8 . . .” |
Implications
The CEPI-Novavax contract contains several safeguards. In addition to some transparency around pricing and supply, Novavax has committed to reasonable pricing—although the term is vaguely defined, and the enforcement mechanisms remain unclear.[60] While total allocation remains ambiguous, Novavax has also committed to providing the doses produced under this contract to COVAX.
CEPI also has a worldwide and royalty-free license (“Public Health License”) to intellectual property needed to produce NVX-CoV2373. CEPI can exercise its license if it is not in material breach of the agreement, the vaccine has been authorized by at least one regulatory agency, and one of several conditions has occurred, including if the corporation has breached the Equitable Access Plan,[61] or declines to expand its work with CEPI upon request. If CEPI chooses to exercise the license, Novavax, in part, must transfer project materials, project results and know-how needed to accomplish the project activities to a designated third-party (“Trusted Collaborator”). While Novavax initially has the ability to name the party, if negotiations fail, CEPI can unilaterally appoint a third-party.[62] Novavax has agreed not to sue CEPI or its designee.[63]
Novavax has so far appeared to share technology with some manufacturers, striking deals with SKBioPharma, Takeda and Serum Institute of India among others to expand supply.[64] COVAX should center its equitable access authorities in future negotiations with Novavax and exercise its leverage to expand affordable supply. Should Novavax fail to provide affordable supply or otherwise meet CEPI’s equitable access requirements if the vaccine is approved, CEPI should exercise its public health license.
University of Queensland (CSL)
Overview
CEPI provided the University of Queensland (UQ) with up to $4.5 million in funding to develop its COVID-19 vaccine candidate. The first portion of this funding was allocated to UQ in January for initial research and development of the vaccine.[65] CEPI increased its contribution to UQ in June as part of a partnership with the Australian biotech company CSL to support clinical development and large-scale manufacturing of UQ’s vaccine candidate.[66] Previously, CEPI provided $10.6 million to develop its vaccine platform technology which is based on a “molecular clamp” in 2019.[67]
Equitable Access
In a meeting with CEPI’s Equitable Access Committee, CEPI staff noted that the coronavirus agreement built on the existing partnership.[68] They were thus able “to rely on the equitable access provisions that were agreed upon in the underlying platform-directed agreements.”
In a 2019 document, CEPI described the equitable access conditions in its partnership agreements.[69] The document noted that its UQ partnership had multiple safeguards,[70] including in part:
- Step-in rights;
- Expanding supply through a Trusted Manufacturer; and
- Reporting requirements related to equitable access, cost of goods and supply.
A July press release stated that dose allocation was based on CEPI and CSL’s relative contribution to project costs; doses allocated to CEPI will be distributed through COVAX; and that CEPI also had a right of first refusal for surplus doses beyond CSL’s allocation.[71]
Implications
There is very limited public information available about the agreement between CEPI, CSL, and UQ. CSL appears to be a “Trusted Manufacturer” that will help UQ expand supply and is expected to subcontract with other global manufacturers.[72] CEPI also appears to maintain “step-in” rights that could permit additional manufacturers. So far, Australia appears to be the sole purchaser of the vaccine candidate, having procured 51 million doses.[73] CEPI should use its leverage to expand affordable supply and step-in if necessary.
Moderna
Overview
Moderna has worked with the U.S. National Institutes of Health to develop mRNA-1273. In January, CEPI played an early critical role by providing up to a million dollars for manufacturing preliminary clinical batches of mRNA-1273.[74] This early-stage investment allowed the candidate to rapidly enter Phase I clinical trials run by the NIH. (The U.S. Biomedical Advanced Research and Development Authority then additionally committed to funding advancement of mRNA-1273 to FDA licensure, meaning that public funding has contributed to mRNA-1273’s entire development).[75]
Equitable Access
In a meeting with CEPI’s Equitable Access Committee, CEPI staff noted that the Moderna agreement included a “short section on equitable access and an obligation to develop an equitable access plan with CEPI within the first six months as part of a more complete agreement.” The short section “provided an obligation to abide by CEPI’s EAC [Equitable Access Committee] policy but didn’t include licensing and step in rights, which would be taken into consideration in the more complete agreement.”[76] Moderna did not receive additional funding and a more complete agreement does not appear to have been signed.
As described previously, CEPI’s equitable access policy lays out CEPI’s general commitments, including its principle that
“Equitable access to epidemic vaccines in the context of an outbreak means that appropriate vaccines are first available to populations when and where they are needed to end an outbreak or curtail an epidemic, regardless of ability to pay.”[77] (emphasis original)
Based on public information, it is not possible to identify the binding contractual obligation for Moderna.
Implications
While there is some uncertainty around the precise scope of its legal obligations, Moderna is nonetheless violating a central principle of the equitable access policy. Equitable access is based on three conditions 1) timing (first available); 2) medical need (where they are needed to end an outbreak or curtail an epidemic; and 3) financial neutrality (regardless of ability to pay).
Moderna’s decisions around supply and pricing both raise concerns. First, in July, the Financial Times reported that Moderna “intended to prioritize” access in the US and other high-income countries.[78] Moderna subsequently entered into advance purchase agreements with Canada, Switzerland, Qatar and the United States, but did not announce any public deals with less wealthy countries.[79] Second, this summer, Moderna reported setting a price of $64-74 per course for smaller volume deals—a figure that is unaffordable for much of the world.[80] Moderna thus appears to have prioritized access based entirely on ability to pay. While it may not be remarkable for a private pharmaceutical corporation to prioritize profits, it is remarkable for a corporation that received critical early support from a funding institution to work against the principles of that very institution.[81]
(Moderna has recently announced it will not enforce patents related to its candidate during the pandemic period—a promising step, but one that needs to be followed with other actions, including technology transfer to expand global supply.)[82]
Table 4 – Moderna Public Sales of mRNA-1273
| Purchaser | Doses | Date Announced |
| Canada[83] | 20-56 million (option) | August 5, 2020 |
| Israel[84] | Not disclosed | June 17, 2020 |
| Switzerland[85] | 4.5 million | August 7, 2020 |
| Qatar[86] | Not disclosed | October 26, 2020 |
| United States[87] | 100-500 million (option) | August 11, 2020 |
CEPI should publish its contract with Moderna. It should also publicly demand compliance with its conditions.
Other Candidates
There is extremely limited public information available about CEPI’s rights in the other candidates it funds. The full analysis is presented in the appendix. Key findings include:
- Inovio: The corporation entered into an agreement, like Moderna, that included a “short section on equitable access and an obligation to develop an equitable access plan with CEPI within the first six months as part of a more complete agreement.”[88] The short section “provided an obligation to abide by CEPI’s EAC [Equitable Access Committee] policy but didn’t include licensing and step in rights, which would be taken into consideration in the more complete agreement.”[89] Additional agreements appear to have been signed, but no further public details are available around the terms.
- Clover Biopharmaceuticals, Oxford (AstraZeneca), The University of Hong Kong, Themis/Institut Pasteur/University of Pittsburgh (Merck): The organizations were funded from CEPI’s February call for proposals.[90] That document noted that applicants “must be willing to transfer their vaccine technology to a global network of large-scale manufacturing.”[91] But while willingness to share technology was a condition of applicant eligibility, it is not clear whether CEPI merely negotiated voluntary technology transfer to a limited set of contracting manufacturers or reserved the right to openly share technology.
- Themis/Institut Pasteur/University of Pittsburgh (Merck): When Merck announced its agreement to acquire Themis, it also announced entering into a memorandum of understanding with CEPI and the Institut Pasteur that seemed to track CEPI’s equitable access policy. In particular, the announcement stated
- “In connection with the transaction, Institut Pasteur, CEPI and Merck have entered into a memorandum of understanding that reflects the parties’ commitments to address the COVID-19 pandemic by developing, manufacturing and distributing the vaccine on a global basis and with pricing that makes the vaccine both available around the world and accessible to those who need it, including low-income, middle-income and high-income countries based on the medical need when the vaccine may become available.”[92] (emphasis added).
- The precise scope of the obligations and enforcement mechanisms remain unclear.
CEPI should publish all contracts, and work with the organizations to ensure compliance.
Vaccines for All
Seven billion people may need a coronavirus vaccine.
A proven safe and effective COVID-19 vaccine would be among the most important scientific achievements of this century. But unequal access—whether due to limited supply or price—threatens to diminish the possible benefits. As GAVI, CEPI, and WHO work to supply the world with a vaccine through COVAX, they should live up to their ideals of treating vaccines as public goods.
What would it look like for COVAX to make a different set of choices?
COVAX would champion the public interest. It would commit to transparency, publishing its contracts and assuring the public about the safeguards it has introduced. It would zealously enforce its equitable access obligations, ensuring that public health need, not purchasing power, drove vaccine access. It would push the manufacturers it funds to share know-how with as many other manufacturers as possible, making full use of all available manufacturing capacity in the world. It would work hand-in-hand with the World Health Organization’s COVID-19 technology access pool to prevent the hoarding of scientific knowledge. In other words, COVAX would do everything it could to make sure affordable vaccines were available to everyone as quickly as possible.
The stakes could not be higher.
Appendix
Inovio
Overview
Since January, CEPI has provided Inovio with up to $22.5 million for its COVID-19 vaccine candidate, INO-4800. Through multiple awards, CEPI has helped fund preclinical work and clinical trials in the U.S. and South Korea, delivery device development, and large-scale manufacturing.[93]
Equitable Access
In a meeting with CEPI’s Equitable Access Committee, CEPI staff noted that the Inovio agreement, like the Moderna agreement, included a “short section on equitable access and an obligation to develop an equitable access plan with CEPI within the first six months as part of a more complete agreement.”[94] The short section “provided an obligation to abide by CEPI’s EAC [Equitable Access Committee] policy but didn’t include licensing and step in rights, which would be taken into consideration in the more complete agreement.”[95] Additional agreements appear to have been signed, but no further public details are available around the terms.
Implications
Like Moderna, Inovio appears to have an equitable access obligation, though the scope remains unclear. Inovio has not entered into any public supply deals. CEPI should publish its contract, and work with Inovio to ensure compliance.
Themis (Merck)
Overview
In March, CEPI provided $4.9 million to a research consortium—including Themis, the Institut Pasteur and the University of Pittsburgh—for a coronavirus candidate based on a measles vector platform. The funding aimed to support preclinical work, manufacture, and preparatory work necessary to reach phase I clinical trials.[96]
In May, Merck announced it was acquiring Themis.[97]
Equitable Access
We identified two sources listing equitable access conditions. First, the funding was drawn from CEPI’s February call for proposals.[98] That document noted that applicants “must be willing to transfer their vaccine technology to a global network of large-scale manufacturing.”[99]
Second, when Merck announced its agreement to acquire Themis, it also announced entering into a memorandum of understanding with CEPI and the Institut Pasteur that seemed to track CEPI’s equitable access policy. In particular, the announcement stated
In connection with the transaction, Institut Pasteur, CEPI and Merck have entered into a memorandum of understanding that reflects the parties’ commitments to address the COVID-19 pandemic by developing, manufacturing and distributing the vaccine on a global basis and with pricing that makes the vaccine both available around the world and accessible to those who need it, including low-income, middle-income and high-income countries based on the medical need when the vaccine may become available.[100] (emphasis added)
Implications
CEPI appears to have attached some equitable access conditions to the Themis (Merck) candidate. The particular contours of the conditions, along with possible enforcement mechanisms, remain largely unclear. For example, while willingness to share technology was a condition of applicant eligibility, it is not clear whether CEPI merely negotiated voluntary technology transfer to a limited set of contracting manufacturers or reserved the right to openly share technology. It is also not clear how the corporation’s compliance with its pricing and supply obligations will be determined. In addition, the Merck memorandum of understanding may also have superseded the initial agreement and eliminated some requirements.
CEPI should publish its contract and memorandum of understanding, and work with Themis (Merck) to ensure compliance.
Clover Biopharmaceuticals
Overview
Following earlier investments in April and July, CEPI announced in November that it would provide Clover Biopharmaceuticals up to $328 million in funding for its COVID-19 S-Trimer vaccine candidate. Together, this funding aimed to support the development of the candidate through to licensure and manufacturing scale-up.[101]
Equitable Access
The initial funding was drawn from CEPI’s February call for proposals.[102] That document noted that applicants “must be willing to transfer their vaccine technology to a global network of large-scale manufacturing.”[103] In addition, in its November funding announcement, CEPI said that it anticipated that the vaccine output funded by CEPI’s investment will be made available for procurement through COVAX.
Implications
Details remain scarce about the arrangement. For example, while willingness to share technology was a condition of applicant eligibility, it is not clear whether CEPI merely negotiated voluntary technology transfer to a limited set of contracting manufacturers or reserved the right to openly share technology. CEPI should publish its contract and work with Clover to ensure compliance.
Oxford (AstraZeneca)
Overview
CEPI provided AstraZeneca and Oxford with up to $384.1 million in funding for their COVID-19 vaccine candidate, AZD1222. This contribution was granted in two parts: an initial grant to Oxford in March supporting preclinical and phase 1 testing ($1.1 million) and a subsequent payment to AstraZeneca in June supporting the technology transfer of AZD1222 production capabilities to manufacturing sites across Europe ($383 million).[104]
Equitable Access
The initial funding for the first deal was drawn from CEPI’s February call for proposals.[105] That document noted that applicants “must be willing to transfer their vaccine technology to a global network of large-scale manufacturing.”[106]
As part of the second deal, CEPI secured 300 million doses for COVAX (150 million courses) on a not-for-profit basis during the pandemic.
Implications
Oxford (AstraZeneca) have publicly said the vaccine will be sold on a not-for-profit basis during the pandemic period. They also appear to have transferred technology to several manufacturers, including those based in Argentina, China, India, Japan, Mexico, South Korea, and Russia.[107] They have not, however, shared know-how publicly through the World Health Organization’s CTAP.
The precise contours of their obligations with respect to CEPI remain unclear. CEPI should publish its contract and work with Oxford (AstraZeneca) to ensure compliance, including by verifying pricing claims.
University of Hong Kong
Overview
In March, CEPI provided the University of Hong Kong with $620,000 in funding for preclinical testing of its COVID-19 vaccine candidate.[108]
Equitable Access
The initial funding was drawn from CEPI’s February call for proposals.[109] That document noted that applicants “must be willing to transfer their vaccine technology to a global network of large-scale manufacturing.”[110]
Implications
The candidate still remains in preclinical testing. Other details remain scarce. For example, while willingness to share technology was a condition of applicant eligibility, it is not clear whether CEPI merely negotiated voluntary technology transfer to a limited set of contracting manufacturers or reserved the right to openly share technology. CEPI should publish its contract and work with the University of Hong Kong to ensure compliance.
[1] CEPI to fund three programmes to develop vaccines against the novel coronavirus, nCoV-2019, CEPI (Jan. 23, 2020), https://tinyurl.com/wqkzcq8. It is not clear whether the Equitable Access Committee had knowledge of the investments at the time of the meeting.
[2] CEPI Equitable Access Committee Meeting Minutes, CEPI (Jan. 17, 2020), https://tinyurl.com/y3kdhnws
[3] Id.
[4] Id.
[5] Gavi: The Vaccine Alliance, https://www.gavi.org/covid19/covax-facility
[6] Id.
[7] Traditionally, CEPI has provided manufacturers direct grants and financing (“push funding”). GAVI uses advance purchase agreements (“pull funding”). COVAX is a collaboration. COVAX, COVAX Facility Terms and Conditions for Self-Financing Participants (Page 14: “Gavi and CEPI are partners within COVAX and are collaborating on the design and operation of the COVAX Facility. This collaboration includes, but is not limited to, engagement with manufacturers, active management of a portfolio of vaccine candidates, and a coordinated approach to incentivizing and securing supply.”)
[8] CEPI Equitable Access Committee Meeting Minutes, CEPI (May 26, 2020), https://tinyurl.com/y4ddhgct. According to CEPI’s Equitable Access Committee (EAC), “as COVAX will not be a legal entity, CEPI will continue to keep legal responsibility for our partnering agreements. Hence, the EAC will maintain responsibility for the equitable access elements.”
[9] COVID-19 technology access pool, WHO, https://tinyurl.com/ybavhba6
[10] COVAX Facility Explainer – Participation Arrangements for Self-Financing Economies, COVAX. Self-financing countries can participate in two ways: through a Committed Purchase Agreement or an Optional Purchase Agreement. The Optional Purchase agreement gives countries the choice to decide if they would like to purchase doses from a manufacturer. Both agreements require an upfront payment, based on the percentage of their population countries select to cover. They also require a final payment to the manufacturer if the doses are approved.
[11] How much co-financing will be required by these countries is yet to be announced.
[12] WHO Member States Briefing, COVAX (Aug. 13, 2020)
[13] The COVAX Facility Question & Answer for Prospective Participants, COVAX. (“The total amount allocated to each Participant over time may be more or less than 20% depending on the funding that is made available as the provision of doses from the two financing streams are separate.”)
[14] Timing is also a concern. According to a senior South African official, some pharmaceutical corporations have indicated that “that they would first service their bilateral agreements [with other countries] and then pass the excess stock on to Covax. So you may end up with a shortage if you’re sitting in Covax. . . “ Coronavirus: Could smaller nations lose out in global vaccine program? The Independent (Sep. 18, 2020), https://tinyurl.com/yyxwsbs4.
[15] Lea Frederiksen et al., The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies, Front. in Immunology (July 21, 2020) (“Based on this estimate, ~5.3 billion vaccine doses are required for a single-dose vaccine, or possibly 12–16 billion in case of a multi-dose vaccine.”)
[16] COVAX, The COVAX Facility Question & Answer for Prospective Participants (“We are encouraging technology transfer to economies which have the capacity to produce vaccines, facilitating introductions and pairing where this is helpful, and where resources permit providing financial support to enable such processes.”)
[17] Based on proxy data and manufacturer engagement data that remains private. Assuming 2 doses per course. The cost per dose for donor-supported countries to participate remains unclear.
[18] COVAX, COVAX Facility Explainer – Participation Arrangements for Self-Financing Economies (“The Facility will endeavor to negotiate the best possible pricing from manufacturers that is lower than or at least no higher than pricing manufacturers have agreed in bilateral deals. The cost per dose will vary by vaccine and manufacturer and the Facility will pass-through the actual, negotiated price to participants. The agreed deals negotiated between the Facility and the manufacturers will dictate the final price of the vaccine and whether the pricing structure is flat/single or tiered.”)
[19] Id.
[20] AstraZeneca takes next steps towards broad and equitable access to Oxford University’s COVID-19 vaccine, AstraZeneca (Jun. 4, 2020), https://tinyurl.com/y2ke7yee
[21] Our Portfolio, CEPI, https://cepi.net/research_dev/our-portfolio/
[22] Draft Landscape of COVID-19 candidate vaccines, WHO (Nov. 12, 2020), https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
[23] Coronavirus vaccine ‘for all’ unlikely before 2022, says leading figure, The Telegraph UK (Oct 1., 2020), https://www.telegraph.co.uk/global-health/science-and-disease/coronavirus-vaccine-unlikely-2022-says-leading-figure/ (CEPI CEO noting that vaccine development effort still needs ~$800 million, and vaccine manufacturing $5 billion).
[24] This built on a previous CEPI MERS coronavirus vaccine partnerships with Inovio.
[25] Our Portfolio, CEPI, https://cepi.net/research_dev/our-portfolio/
[26] This built on a previous CEPI MERS coronavirus vaccine partnership with University of Queensland.
[27] CEPI Third Party Code, CEPI. (“Third Parties contracted to support CEPI funded programs shall comply with the CEPI policies applicable to the work being performed, as published on www.cepi.net, including specifically: Equitable Access Policy. . .”). See also February Call for Funding, CEPI. (“Before submitting an application, applicants should take note of two key award conditions. The first is that each awardee must adhere to CEPI’s policies, which can be found on CEPI’s website. The second is that any funding is dependent on the signing of an award agreement, which provides the terms and conditions under which the award will be made. CEPI is committed to achieving equitable access to all CEPI-supported programmes including vaccines, platforms, data, results, and materials. Specifically, equitable access to epidemic vaccines in the context of an outbreak means that appropriate products are first available to populations when and where they are needed to end an outbreak or curtail an epidemic, regardless of ability to pay. To ensure that CEPI delivers on its commitment to equitable access, CEPI must include access considerations as a component of any agreement with an awardee. Applicants unable or unwilling to meet these requirements should not submit an application”)
[28] Equitable Access Policy, CEPI (2019), https://tinyurl.com/y468ar3e
[29] Id.
[30] Finding equipoise: CEPI revises its equitable access policy, 38 Vaccine 4 (Jan. 28, 2020) https://www.sciencedirect.com/science/article/pii/S0264410X19317190#b0005
[31] CEPI Template Agreement, https://tinyurl.com/yyqgz55y
[32] Our Portfolio, CEPI, https://cepi.net/research_dev/our-portfolio/. Also see CureVac, SEC F-1 Registration Statement, CureVac (Jul. 24, 2020), https://curevac.gcs-web.com/node/6171/html
[33] Id.
[34] CEPI Equitable Access Committee Meeting Minutes, CEPI (Feb 13. 2020), https://cepi.net/wp-content/uploads/2020/04/Minutes-13-02-2020-EAC-Meeting.pdf (“The University of Queensland and Curevac are developing platforms for rapid response. It was noted that the existing partnering agreements include a provision for an additional work package to be developed, so the Secretariat were able to rely on the equitable access provisions that were agreed upon in the underlying platform-directed agreements”).
[35] Id. See, Work Package Statement, Development of CureVac Outbreak Response To Novel Coronavirus (2019-nCoV), SEC (Jan. 27, 2020), https://tinyurl.com/yxc9gzop (“The terms and provisions of the Partnering Agreement are incorporated by reference to this Work Package Statement and all rights and obligations arising under this Work Package.”), & CEPI-CureVac Agreement Article 1.3, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7 (“Any provision in this Agreement referring to “Project Vaccine” will apply respectively to any Product which the Parties will agree to develop under an Additional Work Package.”)
[36] This is not an exhaustive list. The agreement also contains requirements for data, sample, and commercial benefit sharing, as well as a requirement to respond to new outbreaks. See CureVac, SEC F-1 Registration Statement, SEC (Jul. 24, 2020), https://curevac.gcs-web.com/node/6171/html.
[37] Article 7.11, CEPI-CureVac Agreement, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7
[38] Article 7.10. “In the event that the Parties are unable to reach agreement on the identity, number and/or necessary aggregate capacity of Trusted Manufacturers, the matter shall be resolved in accordance with the dispute resolution procedure set out in Clause 22. The contract defines “Trusted Manufacturer” as a “Third Party nominated by the Partner and appointed by CEPI, or nominated by CEPI and appointed by the Partner if so agreed in Additional Work Package Statements.” Affected Territory refers to “the geographic area of any country where there is an Outbreak or that is at risk of an Outbreak taking into account epidemiological data, travel and migration patterns and the lack of availability of other products or product candidates.”
[39] Related to material breach of agreement and default.
[40] Article 1.1.69, CEPI-CureVac Original Agreement. Technology Transfer Materials refers to the “the materials required to be made available to a Trusted Manufacturer to enable such Trusted Manufacture to (i) adapt, develop and use the Platform for the Manufacture of Products for use in the Field and in the Affected Territories (ii) develop, formulate, recreate and show equivalence (where relevant) to Products developed by Partner under an Additional Work Package.”
[41] Article 1.1.53, CEPI-CureVac Original Agreement. Project Technology refers to “technology that is created, devised or arises out of the undertaking and performance of any Work Package/Additional Work Package, the activities set out in the IPDP and any other activities undertaken and performed pursuant to this Agreement.” Article 1.1.27, CEPI-CureVac Original Agreement. Field refers to the diagnosis, prevention and treatment of infections caused by a range of pathogens, including those listed in the “WHO R&D blueprint priority” as updated from time to time. The latest blueprint includes the novel coronavirus. (The pathogens covered also include “novel or previously unrecognized pathogens likely to result in an Outbreak or risk of an Outbreak”, with some limited exceptions in cases where there is commercial interest. However, given that SARS-CoV-2 is listed under the blueprint priority, and that CEPI has already provided money to CureVac for SARS-CoV-2, we assume that the project is within the Field.) See also CureVac, SEC F-1 Registration Statement, SEC (Jul. 24, 2020) https://curevac.gcs-web.com/node/6171/html.
[42] Article 15.1, CEPI-CureVac Agreement, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7. See also CureVac, SEC F-1 Registration Statement, SEC (Jul. 24, 2020) https://curevac.gcs-web.com/node/6171/html (“Furthermore, certain of our patents and technology, including patents and technology relating to our rotavirus, malaria, Lassa virus and SARS-CoV-2 product candidates, were funded in part by grants from nonprofit third parties, including the Bill & Melinda Gates Foundation and CEPI. We are required to fulfill certain contractual obligations with respect to products created using such grant funding. . .”)
[43] We assume technology related to the candidate was developed under the CEPI agreement. Id. See CureVac, SEC F-1 Registration Statement, SEC (Jul. 24, 2020) https://curevac.gcs-web.com/node/6171/html.
[44] Equitable Access Policy, CEPI (2019), https://tinyurl.com/y468ar3e
[45] Since the WHO, CEPI, GAVI allocation mechanism was developed after the CureVac contract was signed, there remains some ambiguity about its applicability. However, if the mechanism is considered a CEPI policy, CureVac may still have an obligation to comply. Fair allocation mechanism for COVID-19 vaccines through the COVAX Facility, WHO, Sept 9. 2020 https://tinyurl.com/yycabvbs (“The fair allocation of vaccines will combine the principle of fairness to meet the basic needs of all countries at the same time in the initial stages (that is, based on proportional allocation), as well as the principle of equity to account for differences in risk profiles across countries”).
[46] The agreement does not specify on what criteria CEPI could withhold its consent. It also introduces some ambiguity in articles 20.9 and 20.11:
“20.9. Use of Platform and Project Vaccines by Partner. Consistent with other obligations in this Agreement, the Partner may at its discretion continue to use the Project Technology [sic, likely referring to Platform and Project Vaccines] for any purpose. . .
20.11. Project Technology. Subject to Clauses 13 and 20, the Partner may at its discretion use the Project Technology for any purpose”
Clause 20.11 is not directly subject to clause 15. But it is still subject to clause 20, which suggests that the use has to be consistent with other obligations in the Agreement.
[47] Article 22.1, CEPI-CureVac Agreement, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7 (“In respect of disputes relating to the validity or infringement of Technology; anti-trust, anti-monopoly or competition law or regulation; and/or breach or threatened breach of Clauses 14, 15 and 17, the Parties irrevocably submit to the exclusive jurisdiction of the Courts of England and Wales.”)
[48] Article 19, CEPI-CureVac Agreement, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7
[49] Article 7.9, CEPI-CureVac Agreement, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7
[50] Article 7.10, CEPI-CureVac Agreement, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7 (“In the event that the Parties are unable to reach agreement on the identity, number and/or necessary aggregate capacity of Trusted Manufacturers, the matter shall be resolved in accordance with the dispute resolution procedure set out in Clause 22.”) and Article 22 (“If the Parties are unable to resolve such dispute through such negotiations within [*****] of such dispute being escalated to the Senior Officers, then in respect of any dispute, controversy or claim other than those that concern: the validity or infringement of Technology; anti-trust, anti-monopoly or competition law or regulation; and/or breach or threatened breach of Clauses 14, 15 and 17, the Parties irrevocably submit to arbitration in accordance with Clause 22.2.”).
[51] Article 11.1, CEPI-CureVac Agreement, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7
[52]CureVac confirms that CEPI retains a license in its financial statements. CureVac F-1, SEC (July 24, 2020), https://curevac.gcs-web.com/node/6171/html. (“In certain scenarios, including if we fail to provide Lassa virus, SARS-CoV-2 or future vaccines developed under the CEPI Agreement at prices that comply with CEPI’s equitable access guidelines, we must grant CEPI a license under certain of our background intellectual property and intellectual property developed under the CEPI Agreement to, among other things, develop our automation solution for use in treating such infectious diseases and to develop, manufacture and market such pharmaceutical products for use in geographic areas where there is a disease outbreak.”)
[53] Article 12.3, CEPI-CureVac Agreement, SEC (Feb. 15, 2020), https://tinyurl.com/y3dcjuk7
[54] While global manufacturing capacity for mRNA vaccines may be constrained, technology transfer could help expand this base and drive additional investments.
[55] CEPI extends collaboration with Novavax to advance development and manufacture of COVID-19 vaccine, CEPI (May 11, 2020), https://tinyurl.com/y8n4akhe
[56] HHS, DOD Collaborate with Novavax to Produce Millions of COVID-19 Investigational Vaccine Doses in Commercial-Scale Manufacturing Demonstration Projects, HHS (Jul. 7, 2020), https://tinyurl.com/y9gmpkjh. The award was primarily focused on manufacturing but it also supported clinical trials.
[57] Novavax 10-Q, SEC (Jun. 30, 2020), https://ir.novavax.com/node/14936/html. This is not an exhaustive list.
[58] “Public Health License” refers to a “grant by Awardee to CEPI of all relevant rights under Project Results, Enabling Rights and Background IP for use in the Field by CEPI as described in Clause 13.”
[59] See also, Novavax 10-Q, SEC (Jun. 30, 2020), https://ir.novavax.com/node/14936/html (Note 11: “Under the terms of the CEPI Funding Agreement, among other things, the Company and CEPI agreed on the importance of global equitable access to any vaccines produced pursuant to the CEPI Funding Agreement. Any such vaccines, if approved, are expected to be procured and allocated through global mechanisms under discussion as part of the Access to COVID-19 Tools (ACT) Accelerator, an international initiative launched by the World Health Organization (“WHO”), Gavi the Vaccine Alliance, CEPI and other global non-governmental organizations and governmental leaders in 2020).
[60] General dispute resolution is through arbitration but that may be impracticable in a pandemic setting. Article 20, CEPI-Novavax Agreement (May 11, 2020).
[61] See Table 3 – Select Public Interest Safeguards in Novavax-CEPI Contract for safeguards that constitute the Equitable Access Plan.
[62] Article 13.6, CEPI-Novavax Agreement (May 11, 2020).
[63] Article 13.7, CEPI-Novavax Agreement (May 11, 2020). (“Awardee . . .shall be deemed to have covenanted not to sue CEPI or designee for the exercise of the Public Health License.”). There appears to be another form of dispute resolution envisaged but the provision is redacted: Article 20.4 “If CEPI invokes its rights under a Public Health License under Clause 13, then the Parties shall pursue [***]. However, because of the exigent circumstances in the Outbreak, Awardee agrees that CEPI [***] and the ultimate resolution of any dispute shall be [***].”
[64] Novavax Announces COVID-19 Vaccine Manufacturing Agreement with Serum Institute of India, Increasing Novavax’ Global Production Capacity to Over 2 Billion Doses Annually, Novavax (Sep. 15, 2020), https://tinyurl.com/y3kjmkxk
[65] CEPI to fund three programmes to develop vaccines against the novel coronavirus, nCoV-2019, CEPI (Jan. 23, 2020), https://tinyurl.com/wqkzcq8
[66] The University of Queensland, CEPI and CSL partner to advance development and manufacture of COVID-19 vaccine candidate, CEPI (Jun. 5, 2020), https://tinyurl.com/yxnzhoe8
[67] Id.
[68] CEPI Equitable Access Committee Meeting Minutes, CEPI (Feb 13. 2020), https://cepi.net/wp-content/uploads/2020/04/Minutes-13-02-2020-EAC-Meeting.pdf (“The University of Queensland and Curevac are developing platforms for rapid response. It was noted that the existing partnering agreements include a provision for an additional work package to be developed, so the Secretariat were able to rely on the equitable access provisions that were agreed upon in the underlying platform-directed agreements”).
[69] Advancing Equitable Access to epidemic vaccines through CEPI’s vaccine and platform development agreements, CEPI (2019), https://tinyurl.com/y2h4hdf4
[70] Id at page 8.
[71] The University of Queensland, CEPI and CSL partner to advance development and manufacture of COVID-19 vaccine candidate, CEPI (Jun. 5, 2020), https://tinyurl.com/yxnzhoe8
[72] Id.
[73] Australia to receive first batch of AstraZeneca COVID-19 vaccine in Jan 2021 – PM to say, Reuters (Sep. 15, 2020), https://tinyurl.com/yxemeh32
[74] Moderna 10-K, SEC (2020), https://investors.modernatx.com/node/8911/html (“NIAID plans to conduct IND-enabling studies and the NIH is conducting a Phase 1 open-label clinical trial of mRNA-1273 in the United States. CEPI has agreed to fund the manufacture of the preliminary clinical batches of mRNA-1273 and, if the Phase 1 clinical trial is successful, BARDA has agreed to fund the advancement of mRNA-1273 to FDA licensure.”). https://cepi.net/research_dev/our-portfolio/
[75] Moderna skirts disclosures of coronavirus vaccine costs, Axios (Aug. 5, 2020), https://tinyurl.com/y3nkdks5
[76] Equitable Access Committee Meeting Minutes, CEPI (Feb 13. 2020), https://cepi.net/wp-content/uploads/2020/04/Minutes-13-02-2020-EAC-Meeting.pdf
[77] Equitable Access Policy, CEPI (2019), https://tinyurl.com/y468ar3e
[78] Moderna pitches virus vaccine at about $50-$60 per course, Financial Times (Jul. 28, 2020), https://tinyurl.com/y45yn4hq
[79] See Table 4 – Moderna Public Sales of mRNA-1273.
[80] Business Updates and Second Quarter 2020 Financial Results, Moderna (Aug. 5, 2020), https://investors.modernatx.com/static-files/4eab8879-d00a-4673-acf7-a0e5943746c2
[81] Upfront deals with select countries also violate the distributional principles established by WHO, CEPI and GAVI for COVAX vaccine access, which in part require proportional allocation to meet the initial basic needs of all countries.
[82] Moderna Vows to Not Enforce Covid-19 Vaccine Patents During Pandemic, Wall Street Journal (Oct 8. 2020), https://www.wsj.com/articles/moderna-vows-to-not-enforce-covid-19-vaccine-patents-during-pandemic-11602154805?eType=EmailBlastContent&eId=a183c7b5-c056-46e9-b11a-c981b106ade3
[83] Canada signs deals with Pfizer, Moderna for experimental COVID-19 vaccines, Reuters (Aug. 5, 2020), https://tinyurl.com/y4kosuuw. See also Canada Exercises Increased Option for 20 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273), Moderna (Sep. 22, 2020), https://tinyurl.com/y4snuelg.
[84] Israel signs agreement with Moderna for potential COVID-19 vaccine, Reuters (Jun. 17, 2020), https://tinyurl.com/y38nepq5
[85] Swiss government signs agreement with Moderna for COVID-19 vaccine, Reuters (Aug. 7, 2020), https://www.reuters.com/article/us-health-coronavirus-swiss-idUSKCN25313R
[86] Moderna announces supply agreement with Qatar (Oct. 26, 2020), https://www.businesswire.com/news/home/20201026005485/en/
[87] Trump Administration collaborates with Moderna to produce 100 million doses of COVID-19 investigational vaccine, HHS (Aug. 11, 2020), https://tinyurl.com/y6co4s8m
[88] Equitable Access Committee Meeting Minutes, CEPI (Feb. 13, 2020), https://tinyurl.com/y4g547nd
[89] Id.
[90] CEPI expands investment in COVID-19 vaccine development, CEPI (Mar. 10, 2020), https://tinyurl.com/yylt768w; CEPI collaborates with the Institut Pasteur in a consortium to develop COVID-19 vaccine, CEPI (Mar. 19, 2020), https://tinyurl.com/yybcest9; CEPI announces COVID-19 vaccine development partnership with Clover Biopharmaceuticals’ Australian Subsidiary, CEPI (Apr. 27, 2020). https://tinyurl.com/y49o4p9t. CEPI partners with University of Hong Kong to develop COVID-19 vaccine, CEPI (Mar. 18, 2020), https://tinyurl.com/y5ff5wo8
[91] Call for Proposals, Proven vaccine technologies, applicable for large scale manufacturing, for rapid response against novel coronavirus, 2019-nCoV, CEPI (2020), https://cepi.net/wp-content/uploads/2020/01/Call-text_CfP2_019-nCoV_30.01.2020-1.pdf.
[92] Merck to Acquire Themis, Merck (May 26, 2020), https://tinyurl.com/y5jfshso
[93] Inovio 10-Q, SEC (Jun. 30, 2020), https://tinyurl.com/yy6utjjx. (Page 28: “In January 2020, CEPI awarded the Company a grant of up to $9.0 million to develop a vaccine against COVID-19. This initial CEPI funding is intended to support preclinical and clinical development through Phase 1 human testing in the United States of INO-4800, the Company’s COVID-19 vaccine candidate against COVID-19. In April 2020, CEPI awarded the Company a grant of $6.9 million to work with the International Vaccine Institute (“IVI”) and the Korea National Institute of Health (“KNIH”) to conduct clinical trials of INO-4800 in South Korea, a grant of $5.0 million to accelerate development of the Company’s nextgeneration intradermal electroporation device, known as CELLECTRA® 3PSP, for the intradermal delivery of INO-4800, and a grant of $1.3 million to support large-scale manufacturing of INO-4800.”)
[94] Equitable Access Committee Meeting Minutes, CEPI (Feb. 13, 2020), https://tinyurl.com/y4g547nd
[95] Id.
[96] CEPI collaborates with the Institut Pasteur in a consortium to develop COVID-19 vaccine, CEPI (Mar. 19, 2020), https://tinyurl.com/yybcest9
[97] Merck to Acquire Themis, Merck (May 26, 2020), https://tinyurl.com/y5jfshso
[98] CEPI collaborates with the Institut Pasteur in a consortium to develop COVID-19 vaccine, CEPI (Mar. 19, 2020), https://tinyurl.com/yybcest9. (“This investment is the result of a recent global call for proposals that CEPI issued in early February, which invited funding applications for proven vaccine technology that could be used to rapidly develop a vaccine against the new coronavirus, and most importantly at scale and with the necessary equitable access provisions.”)
[99] Call for Proposals, Proven vaccine technologies, applicable for large scale manufacturing, for rapid response against novel coronavirus, 2019-nCoV, CEPI (2020), https://tinyurl.com/yxt5m8lj
[100] Merck to Acquire Themis, Merck (May 26, 2020), https://tinyurl.com/y5jfshso
[101] CEPI announces COVID-19 vaccine development partnership with Clover Biopharmaceuticals’ Australian Subsidiary, CEPI (Apr. 27, 2020). https://tinyurl.com/y49o4p9t. CEPI extends partnership with Clover to fund COVID-19 vaccine candidate through global Phase 2/3 study to licensure, CEPI (Nov. 3, 2020), https://cepi.net/news_cepi/cepi-extends-partnership-with-clover-to-fund-covid-19-vaccine-candidate-through-global-phase-2-3-study-to-licensure/
[102] CEPI collaborates with the Institut Pasteur in a consortium to develop COVID-19 vaccine, CEPI (Mar. 19, 2020), https://tinyurl.com/yybcest9. (“This investment is the result of a recent global call for proposals that CEPI issued in early February, which invited funding applications for proven vaccine technology that could be used to rapidly develop a vaccine against the new coronavirus, and most importantly at scale and with the necessary equitable access provisions.”)
[103] Call for Proposals, Proven vaccine technologies, applicable for large scale manufacturing, for rapid response against novel coronavirus, 2019-nCoV, CEPI (2020), https://tinyurl.com/yxt5m8lj
[104] CEPI expands investment in COVID-19 vaccine development, CEPI (Mar. 10, 2020), https://tinyurl.com/yylt768w. CEPI partners with AstraZeneca to manufacture 300 million globally accessible doses of COVID-19 vaccine, CEPI (Jun. 4, 2020), https://tinyurl.com/y8omwkvn.
[105] CEPI expands investment in COVID-19 vaccine development, CEPI (Mar. 10, 2020), https://tinyurl.com/yylt768w
[106] Call for Proposals, Proven vaccine technologies, applicable for large scale manufacturing, for rapid response against novel coronavirus, 2019-nCoV, CEPI (2020), https://cepi.net/wp-content/uploads/2020/01/Call-text_CfP2_019-nCoV_30.01.2020-1.pdf.
[107] AZD1222’s active substance will be produced by mAbxience in Argentina and the final product will be manufactured by the Liomont laboratory in Mexico. The vaccine will then be distributed throughout Latin America, excluding Brazil. Mexico and Argentina Plan to Make AstraZeneca Oxford Vaccine, Bloomberg (Aug. 12, 2020), https://tinyurl.com/y6pk5egz; Shenzhen Kangtai Biological Products agreed to produce 200 million doses of AZD1222 by the end of 2021 as part of their exclusive framework agreement with AstraZeneca. AstraZeneca in first COVID-19 vaccine deal with Chinese company, Reuters (Aug. 6, 2020), https://tinyurl.com/yyktntek; The SII will produce 1 billion vaccine doses for lower and middle income countries. AstraZeneca takes next steps towards broad and equitable access to Oxford University’s COVID-19 vaccine, AstraZeneca (Jun. 4, 2020), https://tinyurl.com/y2ke7yee; Imported doses from AstraZeneca will be supplemented by domestically produced doses of AZD1222 by JCR Pharmaceuticals in Japan. Japan, AstraZeneca agree on 120 mil. COVID-19 vaccine dose supply, Kyodo News (Aug. 7, 2020), https://tinyurl.com/y5s49kfn; South Korea’s SK Bioscience in deal with AstraZeneca on vaccine, Reuters (Jul. 21, 2020), https://tinyurl.com/y4guckvn; R-Pharma will export AZD1222 “to markets in the Commonwealth of Independent States—a nine-country economic group including Russia and post-Soviet republics—the Middle East and the Balkans, an AstraZeneca spokesman said.” AstraZeneca confirms Russia vaccine deal days after COVID-19 hacking accusations surface, Fierce Pharma (Jul. 20, 2020), https://tinyurl.com/y64jx8ya.
[108] CEPI partners with University of Hong Kong to develop COVID-19 vaccine, CEPI (Mar. 18, 2020), https://tinyurl.com/y5ff5wo8
[109] CEPI collaborates with the Institut Pasteur in a consortium to develop COVID-19 vaccine, CEPI (Mar. 19, 2020), https://tinyurl.com/yybcest9. (“This investment is the result of a recent global call for proposals that CEPI issued in early February, which invited funding applications for proven vaccine technology that could be used to rapidly develop a vaccine against the new coronavirus, and most importantly at scale and with the necessary equitable access provisions.”)
[110] Call for Proposals, Proven vaccine technologies, applicable for large scale manufacturing, for rapid response against novel coronavirus, 2019-nCoV, CEPI (2020), https://tinyurl.com/yxt5m8lj